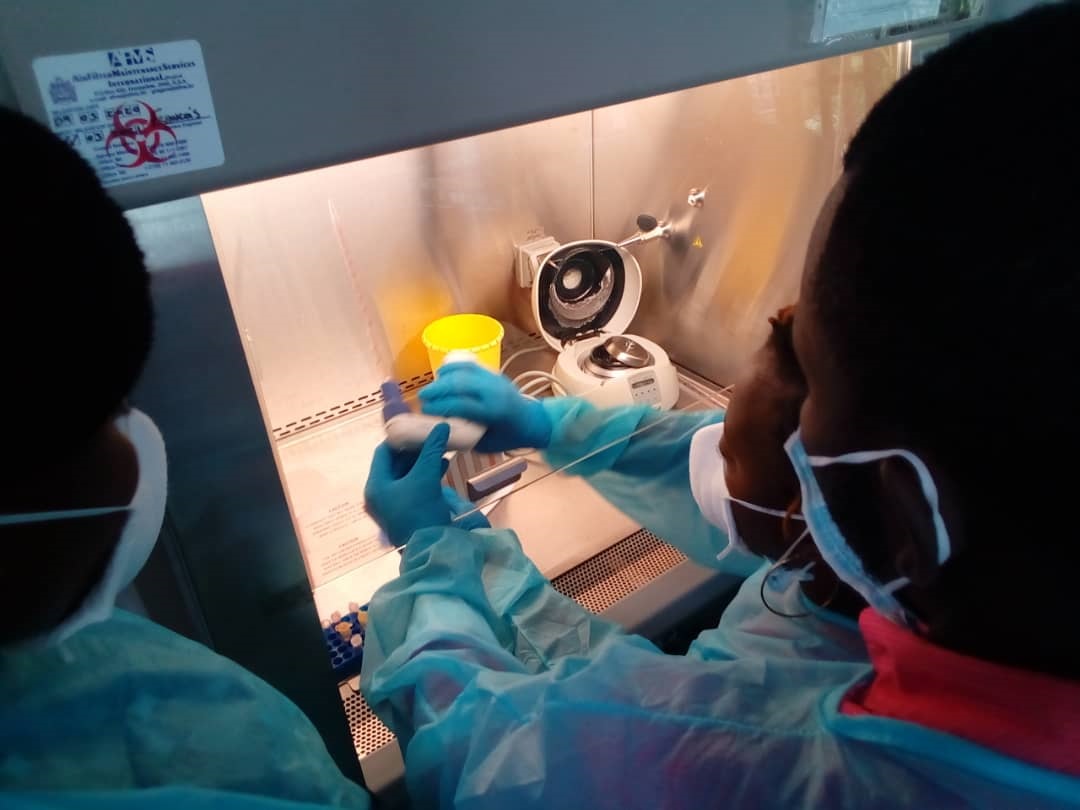
A European-African partnership aimed at improving the ethics and regulatory capacity of clinical research in Central African countries has successfully proposed its project (AfriHetique) to the European Commission (EDCTP). The partnership is coordinated by CANTAM, the Network of Excellence in clinical research for Central African region, lead by Prof. Francine Ntoumi.