R-Evolution Sierra Leone

R-Evolution Worldwide Sierra Leone is a Local Voluntary Organization of Sierra Leoneans established to help and support vulnerable children, youth, women and communities to build a brighter future in changing

R-Evolution Worldwide Sierra Leone is a Local Voluntary Organization of Sierra Leoneans established to help and support vulnerable children, youth, women and communities to build a brighter future in changing

R-Evolution Worldwide Community Interest Company joined in EXCO2019, the first and only global expo dedicated to the innovative solutions provided by the actors of development cooperation: national and international agencies,

R-Evolution Worldwide Community Interest Company participated as observer to the 15th WHO meeting of the Malaria Policy Advisory Committee (MPAC). The meeting highlighted how in the last couple of years

The way to build bridges instead of walls is still long, but I am happy to have got European Union funding for this project. Africlinique project aims to increase the

The ATTENTION project is aimed to improve the access to effective health services for the children in the orphanages and Interim Care Centers (ICCs) of Sierra Leone. Indeed there is

Directorate General Development and Cooperation – EuropeAid was formed on 1 January 2011 following the merger of the EuropeAid Cooperation Office (AIDCO) with the Directorate General for Development and Relations with African, Caribbean

R-Evolution Worldwide – Sierra Leone is starting to operate in the Western Area Urban and Rural District of Freetown, Sierra Leone. R-Evolution Worldwide – Sierra Leone is local community-based organization, registered to
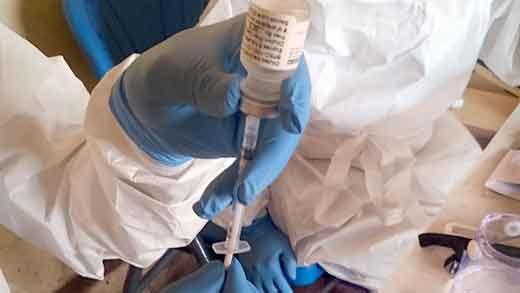
Ebola vaccine candidates are currently being evaluated in phase I–III clinical trials conducted in Africa, the EU and the US. Although preclinical development of candidate vaccines utilise different platforms, including

In a Controlled Human Infection Model (CHIM) study, a well-characterised strain of an infectious agent is given to carefully selected adult volunteers in order to better understand human diseases, how they spread, and