Ebola vaccines at a la glance
January 18, 2019
0 Comments
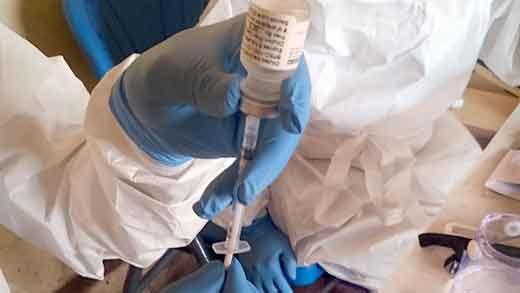
Ebola vaccine candidates are currently being evaluated in phase I–III clinical trials conducted in Africa, the EU and the US. Although preclinical development of candidate vaccines utilise different platforms, including