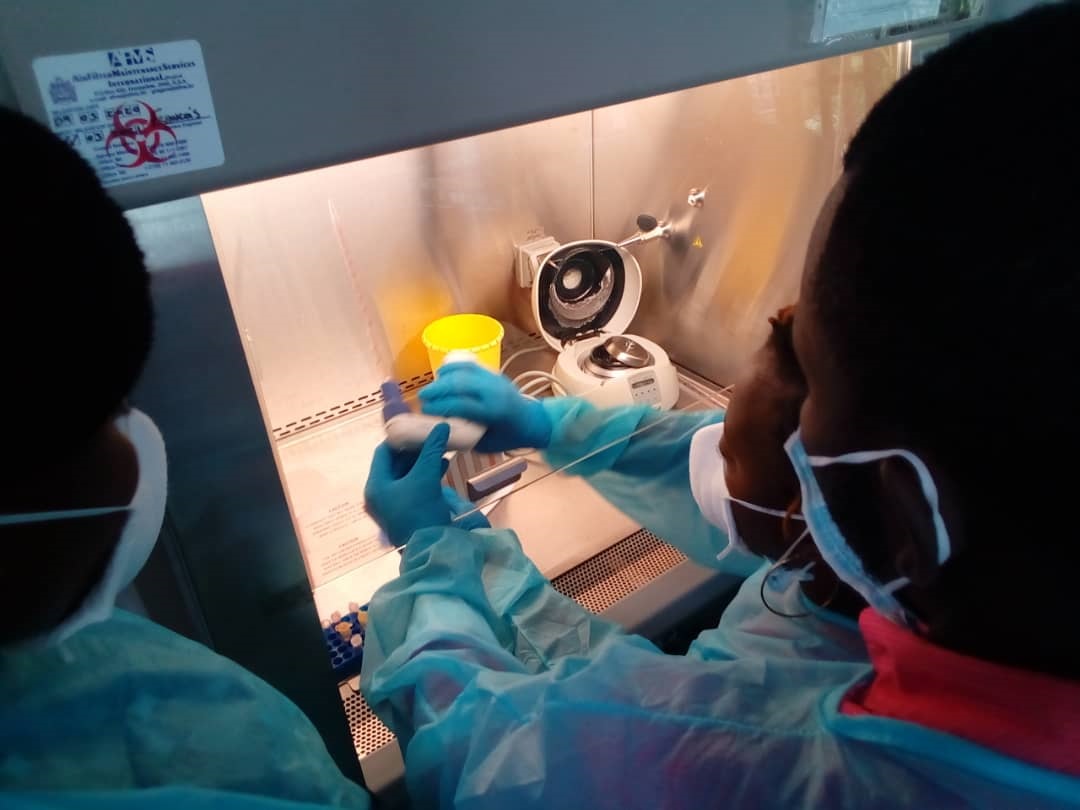
African-European Symposium: Challenges in Research Ethics Assessment, 13:00 – 18:00 CET, May 25th, 2021.

The symposium, organised by The Embassy of Good Science, in collaboration with EUREC, BERC-Luso, AfriEthique, and LiberHetica, aims to facilitate the sharing of experiences and perspectives on research ethics assessment challenges between African and