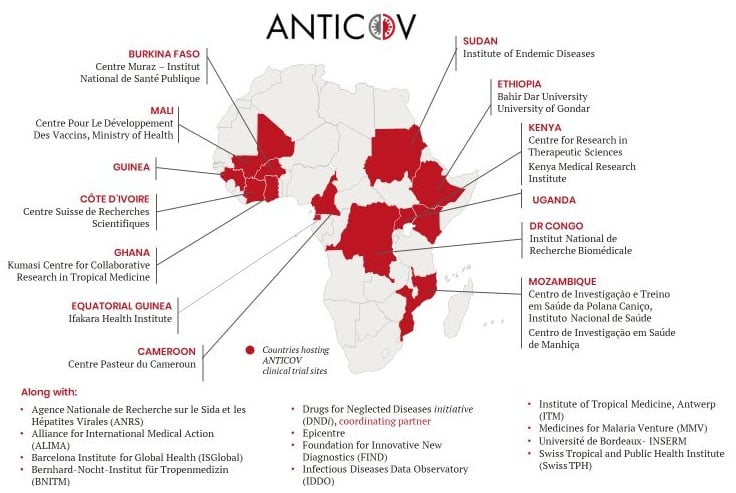
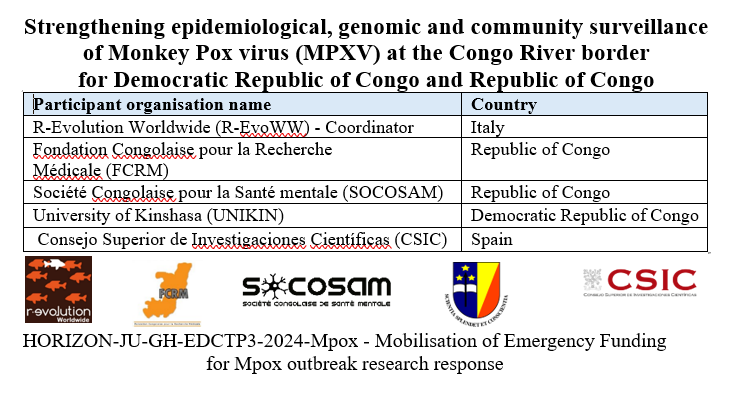
The way to build bridges instead of walls is still long, but I am happy to have got European Union funding for this project. Africlinique project aims to increase the capacity to oversight clinical trials addressing deadly diseases in Central African countries (specifically Republic of the Congo, Democratic Republic of the Congo, Cameroon and Gabon). This achievement was made possible thanks to the exciting proactiveness and invaluable collaboration of a Central African team lead by Prof. Francine Ntoumi, already coordinator of Central Africa Network on Tuberculosis, HIV/AIDS and Malaria (CANTAM).